Introduction
Genus-Species patent applications offer a valuable approach to patent protection, especially in the pharmaceutical domain. By combining a broad genus patent with specific species patents, inventors can secure comprehensive coverage while allowing for future innovations within the invention’s scope. However, to ensure patentability, these applications must adhere to the requirements outlined by the Indian Patents Act, 1970, including novelty, inventive step, industrial applicability, sufficient disclosure, and unity of invention. That means, if the disclosure of the genus patent fails to motivate the person skilled in the art to arrive at the compound of the species patent, then one may seek protection for genus and the species patent separately. However, if the genus patent anticipated the species patent e.g., in the form of Markush structure, then the validity of the species patent claiming the specific compound from the Markush structure cannot be ascertained.
In a recent judgment, BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG v. Defendants. [1-6], by the single judge bench of the Delhi High Court, it has been held that by filing multiple patent claims in respect of the same invention, the plaintiffs attempted to evergreening the invention and re-monopolizing the same and that they failed to make out a prima facie case for grant of interim injunction.
Facts
The case involved six suits filed on behalf of the plaintiff no.1, Boehringer Ingelheim Pharma Gmbh and Co. Kg and its group company, plaintiff no.2, Boehringer Ingelheim (India) Pvt. Ltd, against defendants, Alkem Laboratories, Micro Labs Ltd, Natco Pharma Ltd, Mankind Pharma Ltd, Vee Excel Drugs and Pharmaceuticals Pvt Ltd.
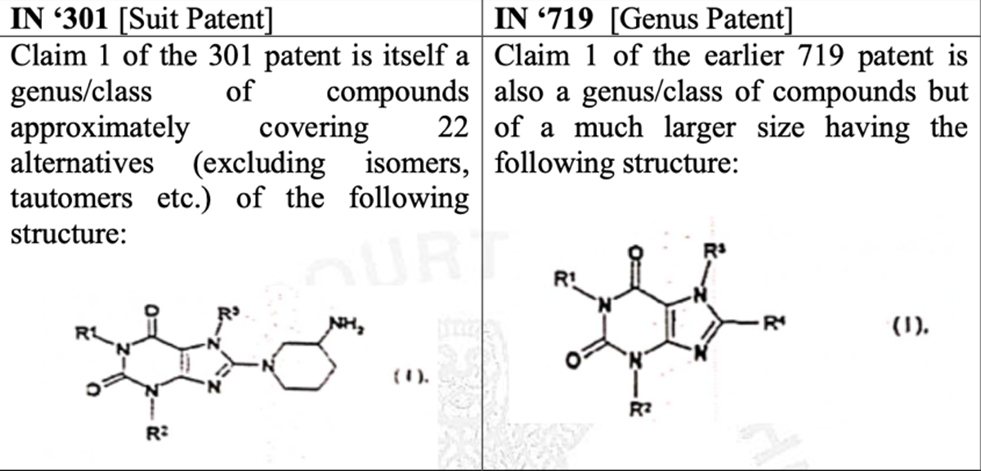
Petitioners seek a permanent injunction to prevent the defendants from infringing their patent titled “8 – (3 AMINOPIPERIDIN–1–YL)-XANTHINE COMPOUNDS”, or “Linagliptin”. An earlier Patent (hereinafter referred to as the “’719 patent”) was granted to the plaintiffs as a genus patent for the Markush formula, which expired in February, 2022.
The plaintiffs contended that Linagliptin per se is neither disclosed nor claimed in the genus patent ‘719 and therefore, a person skilled in the art would not be motivated to arrive at the suit patent from the genus patent. No oppositions against the suit patent were filed. Further, defendants are manufacturing and selling Linagliptin without applying for a voluntary or compulsory license from the plaintiffs.
The Defendants filed a counter claim for revocation of the suit patent of Linagliptin on the grounds that the plaintiffs have filed proceedings against various third parties for infringing the earlier genus patent which covered Linagliptin and its formulations, which amounts to admission that Linagliptin and its formulations were already “protected” and “claimed” in the earlier genus patent., which is now expired. Therefore, the plaintiffs are alleged to be guilty of evergreening the patent.
Issues and Held
- Whether in cases of old patents, the validity of the same must be presumed by the Court? – No.
- Whether the validity of the suit patent must be presumed on account of defendants not having filed pre-grant or post-grant opposition to the suit patent or having filed revocation petition belatedly? – No.
- Whether the defendants have laid a credible challenge to the suit patent? – Yes.
- Whether balance of convenience is in favor of the plaintiffs and against the defendants for the grant of interim injunction? – Balance of convenience tilts in favor of the defendants.
Rationale
- According to Section 13(4) of the Indian Patents Act, the grant of a patent is not prima facie evidence of its validity. Various precedents affirm the same.
- In case of commercial entities, the need to raise challenge arise only on account of commercial realities and necessities. In this case too, the cause of action arose only when the defendants launched their products, and the plaintiffs asserted their rights. A challenge to the validity of the suit patent followed soon after.
-
- Substantial part of the chemical structure in claim 1 of the suit patent and the genus patent are structural similar.
- The plaintiffs have themselves admitted before the Controller of the Indian Patent Office that Linagliptin is one of the possible substitutions of ’719.
- In the ISR, the corresponding PCT publication of ‘719 has been cited as a P, X reference.
- According to AstraZeneca AB & Anr. v. Intas Pharmaceuticals Ltd.[7],
-
- Once a patentee claims infringement of an earlier genus patent in respect of a product, it necessarily follows that the said product was the subject matter of the earlier genus patent.
- The Indian law permits grant of a Markush patent. However, if one of the combinations in the Markush patent includes the product in question, it would form part of the inventive concept of the earlier patent and cannot again be claimed as an inventive concept of a subsequent patent.
-
- In the previous plaints by the Plaintiffs not only in India but also in Canada, it has been averred that Linagliptin is a compound “claimed” and “encompassed” in the genus patent ‘719.
- In its Form 27 submissions (working statements), the company filed the sales details of Linagliptin, for both the genus and species patents.
- Substantial part of the chemical structure in claim 1 of the suit patent and the genus patent are structural similar.
-
- Plaintiffs enjoyed a 20-year monopoly of Linagliptin under the genus patent, while the defendants waited for the patent to expire before launching their drug. Further, the plaintiffs import their product in India while the defendants manufacture their products in India. Suit patent is licensed to Lupin and Eli Lily. Therefore, monetary damages can be calculated and awarded to the plaintiffs and an interim injunction should not be granted.
- Linagliptin is used for the treatment of diabetes, for which the public interest demand easy and affordable access to the drug. The products of the defendants are significantly cheaper than that of the plaintiffs.
- Irreparable injury would be caused not only to the defendants but also to the public if interim injunction is granted in favor of the plaintiff.
Judgement
The Court decided that the suit patent of the plaintiffs is vulnerable to revocation on the ground of prior claiming. Further, by filing multiple patent claims in respect of the same invention, the plaintiffs attempted to evergreening the invention and re-monopolizing the same. Therefore, the Court ordered that there shall be no impediment on the manufacture and sale of products with Linagliptin as the API on account of the suit patent.
Since the plaintiffs failed to pass the three-pronged test of an injunction (prima facie case, balance of convenience, and irreparable injury), all the suits [1-6] for grant of interim injunction were dismissed with costs of Rs. 2,00,000 (approx. 2,381$) awarded to each of the defendant in addition to Rs. 2,00,000 which is awarded in favor of Delhi High Court Legal Services Committee on account of detriment caused to the public interest.
Our Analysis
The court’s decision did not definitively state that a species patent cannot be granted if a genus patent has already been granted. Such a categorical ruling would likely be considered flawed. It is important to recognize that there are specific cases where a drug patent may be granted even if a previous patent or document discloses the drug within a broad Markush structure. The key consideration in such cases is whether the prior patent enables a person skilled in the field to actually produce the drug. This requirement is also well-established for the granting of selection patents, meaning that the prior patent must not provide sufficient information for a skilled person to create the specific product claimed in the selection patent.
In the present case, the plaintiffs had filed multiple patents for different aspects of the same product to extend the term of the patent beyond 20 years term granted by the genus patent, which is impermissible under Indian Patent Law. Although the plaintiff claimed that the species patent was not anticipated by the genus patent, the identical Form 27 were filed for both genus and species patent which made it evident that the plaintiffs had attempted to evergreen its invention and re-monopolize it against the Section 3(d) of the Patents Act 1970 which prevent the extension of patent terms for minor changes or modifications to known substances, thereby enabling generic drug manufacturers to enter the market and reduce the cost of medicines.
Therefore, when dealing with genus and species patents, it is crucial to present the details in Form 27 with great care. Form 27 is a requirement exclusive to India and mandates the disclosure of working details of the patented invention in India. In the case of genus and species patents, it is important to provide accurate and comprehensive information regarding the working of the invention for both the genus and each claimed species. To show the working of genus patent in Form 27, one cannot rely upon the manufacture and sale of same compound of species patent further disclosed in Form 27 of species patent. The Form 27 details should clearly disclose the working of the invention and its embodiments, including any specific compounds or variations. Care should be taken not to disclose the working details of the same components/compounds in both the genus and species patents to avoid any ambiguity or confusion in the disclosure. By presenting the details in Form 27 carefully and accurately, the patent holder can ensure compliance with the legal requirements and strengthen the validity of both genus and species patents.
Conclusion
The important take away from this judgment can be ascertained as follows:
- Two patents cannot be granted for same invention claimed in the form of genus and species patent i.e., evergreening of the patents or layering of patent protection is not permissible under any circumstances.
- There must not be a vast gap between the coverage and the disclosure under the patent.
- Failure to disclose the accurate and comprehensive information on Form 27 may question the validity of the invention.
- Cases where balance of convenience are also squarely applicable, detriment caused to the public interest would cost against the plaintiff rather than causing favor.
In conclusion, multiple patents can indeed be filed for different aspects of a particular product, meeting the criteria of novelty, inventive step, patentability, and industrial applicability. However, granting multiple patents for the same or similar inventions that do not meet the requirements for patentability undermines the spirit of the Indian patent system and can hinder competition and access to new technologies. Therefore, serial patenting, or, layering of protection to evergreen or re-monopolize the invention is generally seen as an abuse of the patent system, and is not permissible.
References
[1] CS(COMM) 239/2019 & CCP(O) 82/2019, I.A. 6797/2019 (O-XXXIX R-1 & 2 of CPC), I.A. 9272/2019 (O-VII R-11 of CPC), I.A. 2042/2020 (u/S 151 CPC), I.A. 2044/2020 (u/s 151 CPC)
[2] CS(COMM) 240/2019 & CCP(O) 81/2019, I.A. 6802/2019 (O-XXXIX R-1 & 2 of CPC), I.A. 9277/2019 (O-VII R-11 of CPC), I.A. 2036/2020 (u/S 151 CPC), I.A.2038/2020 (u/S 151 CPC)
[3] CS(COMM) 236/2022, & I.A. 5801/2022 (O-XXXIX R-1 & 2 of CPC), I.A. 5802/2022(O-XXVI R-9 of CPC), I.A. 5803/2022(O-XI R-1 (6) as amended by the Commercial Court Act), I.A. 5804/2022 (for directions), I.A.22459/2022 (for condonation of delay of 88 days in WS to the CC)
[4] CS(COMM) 237/2022 & I.A. 5806/2022 (O-XXXIX R-1 & 2 of CPC), I.A. 5807/2022 (O-XXVI R-9 of CPC), I.A. 5808/2022 (O-XI R-1 (6) as amended by the Commercial Court Act), I.A. 5809/2022 (for directions)
[5] CS(COMM) 238/2022 & I.A. 5811/2022 (O-XXXIX R-1 & 2 of CPC), I.A. 5812/2022 (O-XXVI R-9 of CPC), I.A. 5813/2022 (O-XI R-1 (6) as amended by the Commercial Court Act), I.A. 5814/2022 (for directions)
[6] CS(COMM) 296/2022 & I.A. 7109/2022 (O-XXXIX R-1 & 2 of CPC), I.A. 7110/2022 (O-XXVI R-9 of CPC), I.A. 7111/2022 (O-XI R-1 (6) as amended by the Commercial Court Act), 7112/2022 (for directions), I.A. 11729/2022 (u/S 151 CPC)
[7] ILR(2009)Supp.(2)Delhi 551

